World Health Organization data show system-wide adverse event reports spanning neurological, cardiac, immune, gastrointestinal, and reproductive categories.

The World Health Organization’s VigiAccess pharmacovigilance database currently lists 5,811,685 individual adverse drug reaction (ADR) reports associated with COVID-19 vaccines as an active ingredient.
A Harvard Pilgrim Healthcare/HHS study confirms fewer than 1% of vaccine adverse events are reported, meaning the number could be closer to half a billion.
These reports are submitted by national drug regulators worldwide and categorized by affected body system.
Below is the full numerical breakdown exactly as listed in the database.
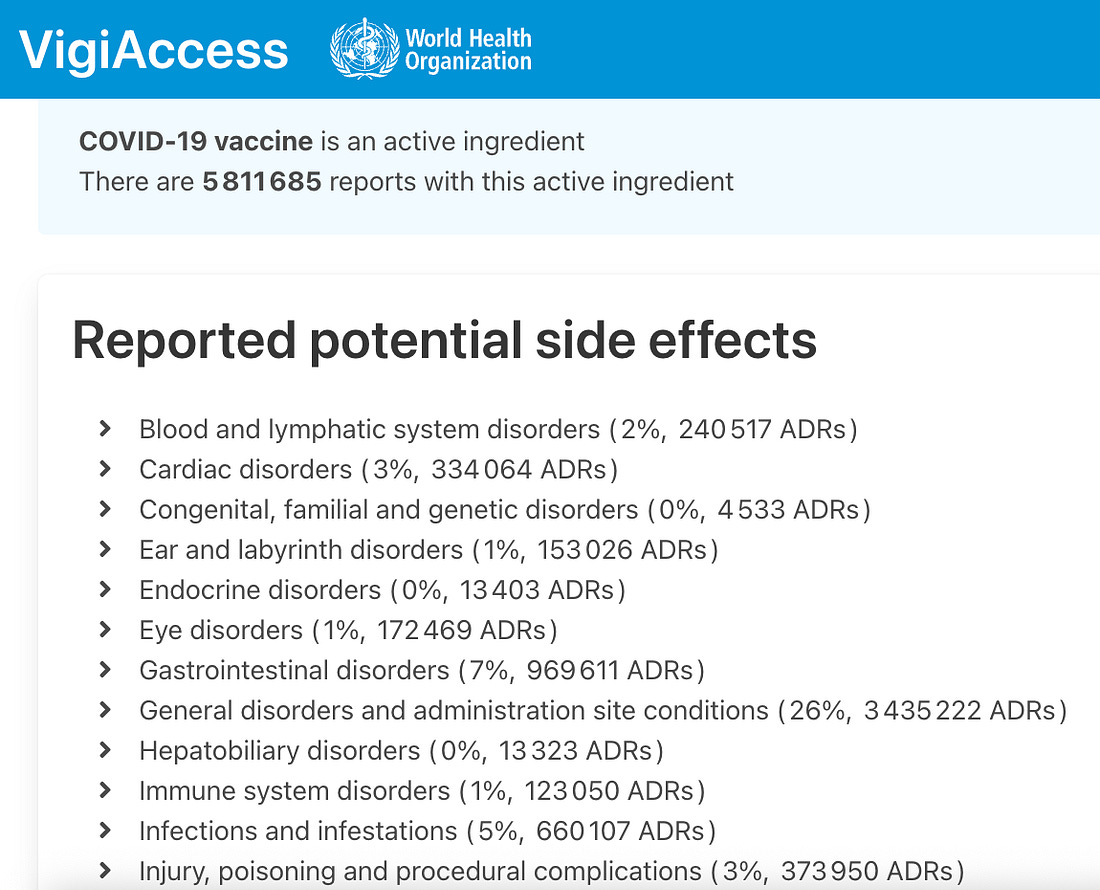
Reported Potential Side Effects by System Category
- General disorders and administration site conditions
3,435,222 reports (26%) - Nervous system disorders
2,162,680 reports (16%) - Gastrointestinal disorders
969,611 reports (7%) - Investigations (laboratory abnormalities, diagnostic findings)
807,850 reports (6%) - Infections and infestations
660,107 reports (5%) - Respiratory, thoracic, and mediastinal disorders
559,163 reports (4%) - Skin and subcutaneous tissue disorders
643,195 reports (5%) - Injury, poisoning, and procedural complications
373,950 reports (3%) - Cardiac disorders
334,064 reports (3%) - Psychiatric disorders
253,443 reports (2%) - Blood and lymphatic system disorders
240,517 reports (2%) - Vascular disorders
245,846 reports (2%) - Reproductive system and breast disorders
280,795 reports (2%) - Musculoskeletal and connective tissue disorders
1,419,363 reports (11%) - Immune system disorders
123,050 reports (1%) - Surgical and medical procedures
121,374 reports (1%) - Metabolism and nutrition disorders
103,797 reports (1%) - Eye disorders
172,469 reports (1%) - Ear and labyrinth disorders
153,026 reports (1%)
Lower-Frequency Categories Still Numerically Significant
- Renal and urinary disorders
47,767 reports - Endocrine disorders
13,403 reports - Hepatobiliary disorders
13,323 reports - Pregnancy, puerperium, and perinatal conditions
14,180 reports - Congenital, familial, and genetic disorders
4,533 reports - Neoplasms (benign, malignant, unspecified)
17,770 reports - Product issues
10,919 reports - Social circumstances
47,909 reports
These figures represent submissions from national health authorities participating in the WHO’s global drug-safety monitoring program.
As of March 2025, 182 health authorities (national pharmacovigilance centers) participate in the WHO Program for International Drug Monitoring.
Each report may include multiple symptoms, meaning totals by category exceed the number of individual reports.
The scale and system-wide distribution of these reports are unprecedented for a single pharmaceutical product class in the VigiBase system.


Leave a comment