Bird Flu Research Explodes 1,000% Worldwide—WHO, CDC, and EcoHealth Lead Rapid Expansion: ‘Journal of Infection and Public Health’. It’s Beyond Ridiculous and Has Been for A Long Time Now!
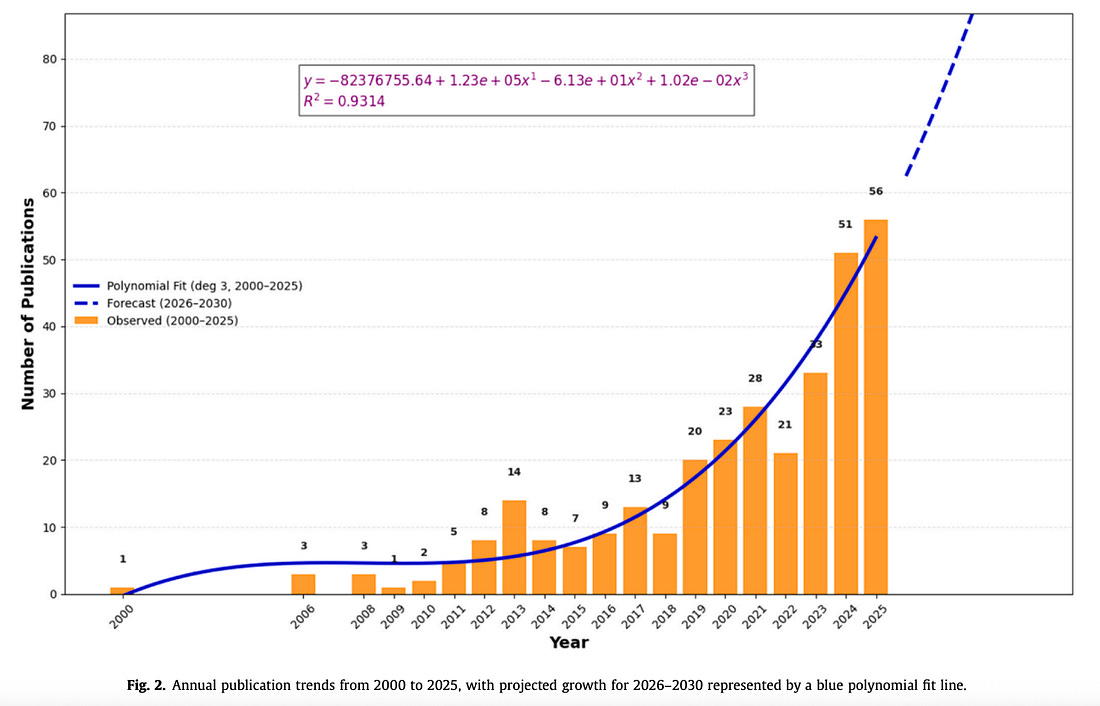
Bird flu publications skyrocket from fewer than 10 papers a year before 2010 to over 50 in 2025—with output expected to hit 111 by 2030, a tenfold surge.

A new Journal of Infection and Public Health paper published this month by Indian Council of Medical Research (ICMR) scientists reveals an unprecedented rise in bird flu–related research worldwide—and predicts that publications on avian influenza will nearly double by 2030, marking what the authors call “accelerating growth” in the field.
The data show that bird flu research output has exploded from fewer than 10 papers a year before 2010 to over 50 in 2025, with the authors projecting a jump to 111 by 2030—a tenfold surge in just two decades, signaling that bird flu has quietly become one of the fastest-expanding areas of global pathogen research.
The figures are based on data from Scopus, a global scientific database that includes most journals indexed in PubMed but extends far beyond biomedical research to cover environmental, veterinary, and policy studies.
This makes Scopus the broadest available measure of the worldwide surge in bird flu–related publications.
The revelation comes as this website has, for years, been raising alarms over the quiet expansion of international bird flu experiments and bird flu pandemic response infrastructure.
The new study, titled “Avian Influenza Research Through the Lens of One Health: A Bibliometric Study” (Elsevier, 2025), analyzed 315 publications on avian influenza between 2000 and 2025 and found that research has exploded since 2018.
The authors expect the trend to continue exponentially over the next five years.
Using a third-degree polynomial model, the team projected that the number of publications will grow from 62 in 2026 to 111 by 2030, with an R² of 0.93 indicating a strong upward trajectory.
“A marked increase occurred after 2018… Forecasts suggest continued growth, with the number of publications expected to rise from 62 in 2026 to 111 in 2030, reflecting increasing research interest and recognition” the paper reads.

The Post-2018 Acceleration
The new study identifies 2018 as the tipping point when H5N1 and One Health publications began to surge.
That timeline aligns with several key developments:
- The 2018–2019 launch of the WHO–FAO–OIE–UN pandemic coordination framework under “One Health.”
- The rollout of avian influenza vaccination programs in China, which reshaped global research priorities.
- The resurgence of EcoHealth Alliance’s field work and U.S. government contracts related to avian flu viruses.
By 2025, the publication rate had risen to 56 papers per year—the highest in two decades
WHO, CDC, and EcoHealth at the Center of the Growth
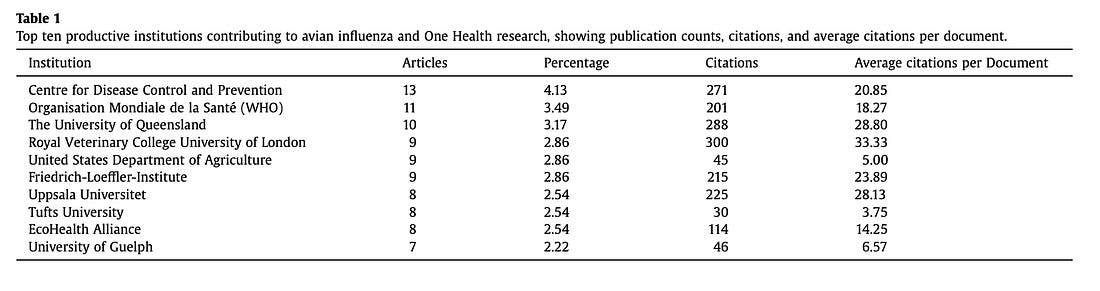
According to the paper’s institutional data, the U.S. Centers for Disease Control and Prevention (CDC) leads the world in bird flu and One Health research output, followed by the World Health Organization (WHO) and EcoHealth Alliance.
EcoHealth is the same organization whose NIH-funded work in Wuhan has been at the center of worldwide controversy over gain-of-function experiments.
Table 1 lists the CDC as having the highest number of publications (13) with 271 citations, followed by the World Health Organization (WHO) (11 publications) and EcoHealth Alliance (8 publications).
In other words, the primary institutions steering the One Health–avian influenza research ecosystem are the same ones historically involved in dual-use virology, pandemic simulation, and cross-species virus manipulation projects.
‘Enhanced Cross-Sectoral Collaboration’—A Code for Expansion
The authors conclude by urging the global scientific community to strengthen “cross-sectoral collaboration” and “sustained surveillance” in poultry and wild birds, warning against “undetected transmission chains in resource-limited settings.”
While framed as disease prevention, this language mirrors the same pandemic-preparedness justification that has fueled massive funding surges into high-containment labs (BSL-2 and BSL-3) and pathogen collection networks around the world.
The study’s repeated emphasis on “biosecurity,” “interdisciplinary cooperation,” and One Health “integration” signals that governments and international bodies are institutionalizing H5N1 work as a standing global priority, not a short-term emergency response.
Normalizing a Permanent Bird Flu Research Pipeline
The study’s authors celebrate this acceleration as a sign of “increasing research interest and recognition.”
But for many observers, it represents something far more concerning—the normalization of a permanent, internationally coordinated pandemic creation and response regime built around H5N1 bird flu.
The report openly ties its findings to global governance structures such as the WHO and the United Nations, stating that the One Health framework is essential for “multisectoral collaboration” and for guiding “policy and research agendas” on avian influenza.
In effect, the paper documents the institutionalization of bird flu research as a permanent fixture of global biosecurity policy—a shift that blurs the line between public health and biodefense, and raises serious questions about how far these programs will go.
Bottom Line
The new Journal of Infection and Public Health study confirms what many have suspected: since 2018, there has been a coordinated expansion of avian influenza research worldwide.
WHO, CDC, and EcoHealth Alliance are leading the charge, and the scientific community now projects that output to double by 2030.
Behind the rhetoric of “One Health” and “collaboration” lies a long-term global infrastructure for studying, modifying, and surveilling avian viruses — one that could easily serve both pandemic prevention and pandemic creation agendas.
The normalization of this permanent H5N1 research pipeline marks the next chapter in the international “pandemic preparedness” agenda — and the public deserves to understand what’s being built, and why.






















Recent Comments